Packs sizes (2)
| code | packaging size | price per unit | box price per unit | |
|---|---|---|---|---|
| Code & packaging | Price per piece | |||

|
code
131340.1211
|
packaging size
1000 g
|
price per unit
single
157,70€
|
box price per unit
134,05€x 6 units
|

|
code
131340.0914
|
packaging size
5 kg
|
price per unit
single
Request a quote
|
box price per unit
|
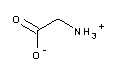
Technical data
- Melting Point:
- 232 °C
- Solubility:
- water 250 g/l at 20 °C
- Physical Description:
- solid
- Product Code:
- 131340
- Product Name:
- Glycine (Reag. USP) for analysis, ACS
- Quality Name:
- for analysis, ACS
- Specifications:
- Minimum assay (Perchl. Ac.): 99.0%
Assay (as N2) (Kjeldahl) (calc. a.d.s.): 18.4-18.8%
Identity: IR passes test
pH of 5% solution: 5.9-6.3
T.L.C.: passes test
Maximum limit of impurities
Insoluble matter in H2O: 0.005 %
Darkened substances by H2SO4: passes test
Loss on drying at 105°C: 0.2%
Residue on ignition (as SO4): 0.05 %
Chloride (Cl): 0.005%
Ammonium (NH4): 0.005%
Sulfate (SO4): 0.005%
Hydrolizable substances: passes test
Heavy metals (as Pb): 0.001%
As: 0,0001 %
Cu: 0,0005 %
Fe: 0,0005 %
Ni: 0,0005 %
Pb: 0,0005 %
- WGK:
- nwg
- Storage:
- Room Temperature.
- Master Name:
- Glycine
- Synonyms Long Text:
- Aminoacetic Acid, Glycocoll
- EINECS:
- 200-272-2
- CS:
- 29224985
Documents
Inquiry
Comments
Glycine or glycocolla (Gly or G) is one of the amino acids that form the proteins of living beings. In the genetic code it is represented by the codons GGU, GGC, GGA or GGG.It is the smallest amino acid and the only non-chiral amino acid of the 20 amino acids present in the cell. Its chemical formula is NH2CH2COOH and its mass is 75.07 g/mol. Glycine is a non-essential amino acid. Another (old) name for glycine is glycocholine.
Glycine acts as an inhibitory neurotransmitter in the central nervous system. It was proposed as a neurotransmitter in 1965.
Glycine is used - in vitro - as a gastric medium, in 0.4 M solution, buffered to stomach pH to determine bioaccessibility of potentially toxic elements as an indicator of bioavailability.
History
Glycine was first isolated from gelatin in 18203 by Henri Braconnot, director of the botanical garden in Nancy.
Shortly after the substance had been named glycocol ('sweet glue') Jöns Jakob Berzelius in 1848 decided to apply a shorter name, glycine, from the Greek γλυκύς, 'sweet taste'. The chemical structure was not correctly described until 1858, which was done by the French chemist Auguste André Thomas Cahours.
Metabolism
Synthesis
Glycine is not essential in the human diet, as the body itself is responsible for synthesizing it. All cells have the capacity to synthesize glycine. There are two ways to synthesize glycine: phosphorylated and non-phosphorylated. The most important precursor is serine. Phosphoserine phosphatase dephosphorylates phosphoserine to serine. The enzyme serine hydroxymethyl transferase gives rise to glycine from serine. Glycine used as a neurotransmitter is stored in vesicles, and is expelled in response to substances.
Industrially it is prepared by a one-step reaction between chloroacetic acid and ammonia.
ClCH2COOH + NH3 → H2NCH2COOH + HCl.
Elimination
The reuptake mechanism is sodium-dependent.
Receptors
Glycine has a receptor (distinct from receptors for GABA) that can also bind β-alanine, taurine, L-alanine, L-serine and proline. It is not activated by GABA. An antagonist is the alkaloid strychnine, which blocks glycine and does not interact with the GABA system. It increases the conductance for chlorine (similar to the glycine receptor to GABA-A).
This receptor has been purified using the alkaloid strychnine. This receptor is a complex of α and β subunits, with pentameric structure, with homology to GABA-A and the nicotinic receptor. It also has 4 transmembrane domains. In the cytosol, they bind to gefirin to anchor to the cytoskeleton. It is thought that other ionotropic receptors may have a similar membrane anchoring system.
Image of the NMDA receptor present in the nervous system. Glycine is also a co-agonist of the NMDA receptor for glutamate. The number 9 indicates its binding site. Meaning of numbers: 1.- Cell membrane. Channel blocked by Mg2+ at the blocking site (3). 3. 3.- Site of Mg2+ blockade. 4.- Binding site of hallucinogenic compounds. 5.- Zn2+ binding site. 6.- Binding site of agonists (glutamate GLU) and/or anti-agonist ligands (APV). 7.- Site of glycosylation. 8.- Proton binding site. 9.- Glycine binding site. 10.- Binding site for polyamines. 11.- Extracellular space. 12.- Intracellular space. 13.- Subunit of the complex.
Glycine is a non-polar amino acid.
Functions
Glycine is used in the organism to synthesize a large number of substances; for example, the C2N group of all purines is obtained thanks to glycine.
Diagram showing how glycine participates in the structure of purines.
It also has functions as an inhibitory neurotransmitter in the central nervous system, especially in the spinal cord, brain stem and retina. The LD50 of glycine is 7930 mg/kg in rats (oral route), and usually causes death by hyperexcitability.
Glycine is necessary for collagen synthesis. Collagen synthesis means a glycine expenditure of more than 15 grams per day, which must be supplied by the daily diet.7
Presence of glycine in space
NASA seems to be confirming the presence of complex organic compounds in space, off Earth. [1]
The new evidence is very strong. As explained to Radio Nacional de Colombia by scientist Jaime Elsila Cook, a member of the Astrochemistry Laboratory of NASA's Solar System Exploration Division.
According to Cook, the Stardust probe flew in 2004 very close to the tail of comet Wild 2 and smeared itself with glycine, a substance indispensable for the origin of life on Earth. Stardust had a mesh that captured these substances and was brought back to Earth in 2006. Scientists began investigations and found that it was indeed the vital substance.
However, there were always doubts, because since it is on Earth, it was thought that the mesh was possibly contaminated since the probe was manufactured. But, finally, they discovered that the glycine did indeed come from the comet.
Cook explained to Radio National that "what we know is that the Earth was hit by many comets millions of years ago and now what we are finding is that these comets could have carried these amino acids that would be a key ingredient in the beginning of life on our planet".
And he commented that "our discovery supports the theory that some ingredients of life arose in space and reached the earth through the impact of meteorites and comets".
Carl Pilcher, director of NASA's Astrobiology Institute, told the EFE news agency that his team's discovery supports the suspicion that space abounds with the basic substances needed to give life, which is why he believes that life may be more common than is believed in the rest of the universe.
The results and a full explanation of the research will be published in the journal Meteorites and Planetary Science.
In 1994, a team of astronomers from the University of Illinois, led by Lewis Snyder, claimed to have found the glycine molecule in space. According to computer simulations and laboratory experiments, glycine was probably formed when pieces of ice containing simple organic molecules were exposed to ultraviolet light. In 2009 NASA confirmed the presence of this molecule in comet Wild 2 thanks to samples obtained by the Stardust probe that were trapped in a special aerogel and subsequently sent to Earth in a descent capsule.



