Packs sizes (3)
| code | packaging size | price per unit | box price per unit | |
|---|---|---|---|---|
| Code & packaging | Price per piece | |||

|
code
258462.0955
|
packaging size
50x20 ml
|
price per unit
single
150,00€
|
box price per unit
|

|
code
258462.0961
|
packaging size
50x40 ml
|
price per unit
single
160,00€
|
box price per unit
|

|
code
258462.0962
|
packaging size
50x30 ml
|
price per unit
single
155,00€
|
box price per unit
|
Technical data
- Density:
- 1.019 kg/l
- Solubility:
- Miscible with water
- Physical Description:
- liquid
- Product Code:
- 258462
- Product Name:
- Histofix ® - Safe Preservative ready to use (CE-IVD) for clinical diagnostics
- Quality Name:
- for clinical diagnostics
- Headline Comment:
- In Vitro Diagnostic medical device class A in compliance to the Regulation (EU) 2017/746
- Specifications:
- Assay (Iodom.): 3.7-4.0 %
pH: 6.8-7.2
Methanol (w/v): 1 - 1.5 %
- Hazard pictograms
-
- Storage:
- Room Temperature.
- Signal Word:
- Danger
- GHS Symbols:
- GHS08
GHS07
- H Phrases:
- H350
H317
H341
H302
- P Phrases:
- P201
P202
P261
P272
P280
P281
P302+P352
P308+P313
P321
P333+P313
P363
P405
P501
- Master Name:
- Histofix ® - Safe Preservative ready to use
- CS:
- 38229000
Documents
Inquiry
Comments
HistologyHistology is the area of biology that studies the composition, structure, and characteristics of the organic tissues of living beings. Histology is closely related to microscopic anatomy, as its study does not stop at tissues, but goes beyond them, also observing cells internally and other corpuscles, and is related to biochemistry, cytology and medicine.
- Histology has become very important in medicine and biology as it is crucial for these disciplines because it’s in between the intersections biochemistry, molecular biology, and physiology on the one hand and pathological processes and their consequences on the other.
- Classical histology techniques are applied in medical laboratories for the diagnosis of a wide variety of diseases. These techniques are adequate in most diagnostic cases. However, when diagnosis with these classical techniques cannot be considered reliable, additional methods must be used. These techniques include histochemical staining, immunohistochemical methods, DNA hybridization, fluorescence in situ hybridization (FISH), PCR, flow cytometry and others.
- The medical laboratories mentioned above focus on primarily on production-based applied science (number of diagnostics tests), as opposed to research laboratories which focus on basic science on an academic basis.
- A medical laboratory or clinical laboratory is the place where tests are usually performed on clinical samples to obtain information about a patient's health in terms of diagnosis, treatment, and prevention of disease. - Research laboratories use conventional techniques for Genomics, Proteomics and Cell Culture procedures.
- PanReac AppliChem branded products for hospital laboratories: - Medical laboratories: Microscopy products.
- Research Laboratories: Genomics, Proteomics and Cell Culture Products. - Medical laboratories
- In many countries there are mainly two types of medical laboratories depending on the type of research performed.
- Hospital laboratories: laboratories integrated into a hospital to perform tests on patients’ samples.
There are 4 different types of laboratories:
• -Clinical pathology laboratories: hematology, histopathology, cytology, routine pathology.
• Clinical microbiology laboratories: bacteriology, mycobacteriology, virology, mycology, parasitology, immunology, serology.
• Clinical biochemistry laboratories: biochemical analyses, hormone assays, etc.
• Molecular diagnostic laboratory or cytogenetics and molecular biology laboratory.
• External clinical laboratories: for extremely specialized tests, the sample can go to an external research laboratory.
- ITW Reagents offers a complete range of products for histology, hematology, and microbiology under the PanReac AppliChem brand, including the most common used reagents in the process of sample preparation for microscopic examination. This range covers all stages of fixation, clearing, paraffin embedding, staining and mounting. ITW reagents also have a wide range of products for research in different areas in Life Sciences for assays to be developed in hospital laboratories: genomics, proteomics, and cell culture.
- Many of the PanReac AppliChem branded products used in microscopy carry the CE IVD marking in compliance with the new European Regulation (EU) 2017/746 on in vitro diagnostic products (IVDR).
- Histological processing techniques and stages
- Although most of the stages of histological processing are common, some of them are unique to a single type of sample processing. For example, embedding is done only on tissues and heat fixation only on blood samples.
- The most common stages and the products used are as follows:
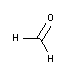
• Fixation: Fixation interrupts degradation processes that occur after cell death, trying to preserve the tissue/cellular architecture and composition as close as possible to how it was in the living organism. The most common type is chemical fixation with Formaldehyde 3.7-4.0% w/v buffered to pH=7 and stabilized with methanol and, for increased safety, Histofix® Formaldehyde single-dose ready-to-use preservative.
• Dehydration and clearing: Dehydration is the complete removal of water from the specimen or tissue sample so that it can be properly embedded in the subsequent non-water-soluble embedding media. The fixed and washed specimens are passed through 96% alcohol and then absolute alcohol for varying lengths of time, typically one and a half hours in each bath. Clearing is the replacement of the drying agent with a substance miscible with the embedding medium to be used. The most common products in this stage are alcohols such as absolute ethanol, 96% ethanol, 70% ethanol, which can be denatured or not, as well as xylene.
• Inclusion: by inclusion, the water in the tissue is replaced by a liquid medium capable of solidifying under suitable temperature conditions. Paraffin wax P.F. 55-58°C plasticized + DMSO in lentils or simply Paraffin wax P.F. 56-58°C in lentils is used for this purpose.
• Cutting: Paraffin-embedded tissues are cut thinly enough (4-6 microns) to allow light to pass through for examination under a microscope. This is done with a microtome: a mechanical instrument used to make micrometric sections of tissue. We recommend our paraffin cleaner to keep the microtome clean.
• Clearing-Hydration: Dewaxing-hydration is the process of removing the embedding medium from paraffin-embedded tissue sections and rehydrating them for proper dye penetration. The most common reagents at this stage are alcohols such as Absolute Ethanol, Ethanol 96%, Ethanol 70% which may or may not be denatured in addition to Xylene.
• Staining: Most tissues, especially those of animal origin, are colorless unless they contain pigment. It is therefore necessary to stain them for microscopic observation. This is achieved using dyes, substances which, in contact with a suitable support, bind to it in a durable way, transmitting its color. The most used dyes are hematoxylin and eosin, but there is a wide variety of stains depending on the tissue to be stained.
• Mounting and immersion: Once the preparations have been rinsed, they must be mounted definitively. Mounting agents can be aqueous or non-aqueous; the type used depends on the relevant protocol. Examples of mounting media are DPX, Canada Balsam and Eukitt®. Immersion media are liquids, often natural oils, which have a defined refractive index. It is important that the refractive index is approximately 1.5 (same index as glass). This allows for homogeneous oil immersion. For this purpose, Immersion Oil or Cedar Oil is used.
• Microscopic analysis: This is the microscopic observation phase. No additional reagents are needed.
FAQs
What is Histology?
Histology is a subject of biology/medicine that studies the composition, structure, and characteristics of the organic tissues of living beings. Histology is closely related to microscopic anatomy, since its study does not stop at the tissues, but goes beyond, also observing the cells internally and other corpuscles, relating to biochemistry and cytology.What is meant by fixation in histology?
In the area of histology, pathology and cell biology, fixation consists of preserving biological tissues from deterioration by autolysis or putrefaction. It stops any ongoing biochemical reaction and can also increase the mechanical strength or stability of the treated tissue. Tissue fixation is a fundamental step in the preparation of histological sections. The overall aim of fixation is to preserve cells and tissue components in such a way that thin and stained sections can be produced. This enables the study of tissue structure, which is determined by the shape and size of macromolecules (in and around cells) such as proteins and nucleic acids.What is meant by autolysis?
The destruction of cells or tissues by their own enzymes, especially those released by lysosomes is called autolysis.What is meant by putrefaction?
Putrefaction is the decomposition of organic matter especially the typically anaerobic splitting of proteins by bacteria and fungi with the formation of foul-smelling incompletely oxidized products. The process of putrefaction is started by the bacteria that are present in the environment. These bacteria will breakdown the organic matter and produce waste products, such as methane and ammonia. These gases will cause the object to swell and release a foul odor.What is a tissue infiltration medium?
A tissue infiltration medium is a special formulation of purified paraffin and plastic polymers for infiltration or embedding the biological sample.How can I remove water from a tissue sample/biopsy?
This process which process? is commonly carried out by immersing specimens in a series of ethanol (alcohol) solutions of increasing concentration until pure, water-free alcohol is reached. Ethanol is miscible with water in all proportions so that the water in the specimen is progressively replaced by the alcohol.Which solvents can I use for the removal of water in tissue samples/biopsies in histology/pathology?
Absolute ethanol, ethanol 96%, ethanol 70 % are the most commonly used solvents. Because melted paraffin wax is hydrophobic (immiscible with water), most of the water in a sample must be removed before it can be infiltrated with paraffin. This process is usually carried out by immersing samples in a series of ethanol (alcohol) solutions of increasing concentration until pure alcohol without water is achieved. Ethanol is miscible with water in all proportions, so that the water in the sample is progressively replaced by the alcohol. A series of increasing concentrations of alcohol is used to avoid excessive deterioration of the tissue.What is meant by graded dehydration series in histology biopsy preparation?
Dehydration is the complete removal process of water from fixed tissue. Graded concentrations of ethanol are used for dehydration; the tissue is immersed in 70% ethanol in water, followed by 96% and 100% solutions. Ethanol ensures total dehydration, making it the reagent of choice for the processing of electron microscopy specimens. For delicate tissue it is recommended that the processing starts in 30% ethanol. Desiccants must be water soluble, and ethanol is the most used dehydrating agent. Ethanol has several special properties for dehydration: complete miscibility with water, hardening effect, high dehydration power and tissue permeability. To avoid excessive tissue stiffness, it is usually necessary to use graded ethanol (usually at successive concentrations of 70%, 80%, 95% and absolute ethanol) to gradually replace water in the tissue. Isopropanol can also be used to replace EtOH, but with changes to standard dehydration protocol. Depending on the size or type of the specimens, one must optimize the time in the graded Isopropanol. Very small samples can overharden, and fatty or larger specimens will need more dehydration. The isopropanol does not mix with paraffin, so to use a clearing agent such as xylene will be needed.What is brightfield microscopy?
Brightfield (BF) microscopy is the simplest of the illumination methods in optical microscopy. The sample is transilluminated with white light (i.e. illuminated from below and viewed from above) and the contrast of the sample is due to the attenuation of transmitted light in the dense areas of the sample. Brightfield microscopy is the simplest of the techniques used to illuminate samples in optical microscopes, and its simplicity makes it a very popular technique. The typical appearance of a brightfield microscopy image is that of a dark sample on a bright background, hence the name.What is meant by dehydrating in histology?
Dehydration is the complete removal process of water from fixed tissue. Graded concentrations of ethanol are used for dehydration; the tissue is immersed in 70% ethanol in water, followed by 96% and 100% solutions. Most infiltration media (the solid support matrix for cutting tissue samples) are hydrophobic, so water must be removed from the tissues before further processing. There are two general methods for dehydrating tissues. The most common method is the gradual replacement of water with an organic solvent by transporting the tissue through solutions of increasing concentration (ethanol, acetone) - this is called a graded dehydration series. A less common method in brightfield microscopy is rapid dehydration with an organic reagent (2-methoxy ethyl acetate, dimethoxy propane, triethyl phosphate). Rapid dehydration quickly replaces the water in the tissue without damaging the surface tension, as can be the case with alcohols or acetone. 2-methoxy ethyl acetate and dimethoxy propane have hydrophilic and hydrophobic regions. This allows the solvent to interact with biological membrane lipids without destroying them. The resulting dehydration occurs through a mass flow mechanism rather than a chemical reaction. The relative benefit of each method is subjective and depends on the tissues involved and personal preference. In the past, rapid dehydration was limited to plastic infiltration procedures, but can certainly be used with paraffin techniques.What is meant by staining in histology?
A stain or dye is an aid used in microscopy to enhance the contrast of the image seen under the microscope. Stains are substances commonly used in biology and medicine to highlight structures in biological tissues that are to be observed using various types of microscopes. Different dyes can be used to increase sharpness and examine large sections of tissue (e.g. highlighting muscle fibers or connective tissue), to highlight cell populations (e.g. sorting different blood cells) or even to highlight organelles within individual cells. In biochemistry, this means adding a specific dye (which selectively binds to either DNA, proteins, lipids, carbohydrates, etc.) to a substrate to qualify or quantify the presence of a particular compound. Both staining and fluorescent labelling can serve the same purpose. Different types of biological dyes are also used to label cells in flow cytometry and to label proteins or nucleic acids in gel electrophoresis.Which stains are used in histology?
The most common stains used in histology are the following: Routine stains: Hematoxylin & Eosin; Special stains: Van Gieson, Toluidine Blue, Alcian Blue, Giemsa, Reticulin, Nissl, Orcein, Sudan Black B. Masson's Trichrome, Mallory's Trichrome, Azan Trichrome, Cason's Trichrome, Periodic Acid Schiff, Weigert's Resorcin and Fuchsin.How can I fix a biopsy?
A biopsy can be fixed with formaldehyde or formalin. This process is called fixation. The purpose of formaldehyde or formalin is to fix the tissue to stop the autolytic changes in the biopsies. All specimens should be placed immediately following excision into 10% neutral buffered formalin to allow them to “fix”. Ideally, the amount of fixative should be at least ten times the volume of the specimen.What is the difference between formaldehyde and formalin?
The difference, basically, formaldehyde is a colorless, water-soluble, flammable gas at room temperature with a sharp, irritating smell. However, formalin is a liquid, which is prepared by mixing formaldehyde gas and water. This is the main difference between formalin and formaldehyde. Generally, a saturated solution of formalin contains about 40% (by volume) or 37% (by weight) of formaldehyde gas and a stabilizer to prevent formaldehyde polymerization.Is formaldehyde carcinogenic?
Formaldehyde is one of the most thoroughly evaluated substances. In 2014 Formaldehyde was reclassified as a Carcinogen Category 1B. The new classification entered into force in June 2014 when published in the Official Journal of the European Union (COMMISSION REGULATION (EU) No 605/2014 of 5 June 2014).Why is ethanol used in histology?
In histology, Ethanol is used as a dehydrating and rehydrating agent. Dehydration serves to prepare the specimen to be embedded in paraffin and to remove the paraffin with the use of xylene as an intermediate solvent. Xylene is not miscible with water. Ethanol 96% contains some water. Therefore, absolute ethanol must be used as a "bridge" between xylene and 96% ethanol.Why is Xylene used in histology?
In histology, Xylene is used for processing and staining tissues. Xylene is preferred for tissue processing because it makes the tissue transparent so that the paraffin can completely coat the tissue. When preparing slides for microscopy, Xylene can remove any paraffin residue from the slides.What is the paraffin technique?
In histology and pathology, by application of the paraffin technique, biopsies are fixed and embedded in wax. This makes the tissue hard, and much easier to cut sections from in the microtome. The sections are then stained and examined with a light microscope.What does Histofix® mean?
Histofix® is the ITW Reagents brand for all the products related to the fixation of histological samples. This includes formaldehyde for regular biopsy fixations, formaldehyde pink for fixation of tiny biopsies and the decalcifiers for fixation of bones.What can I do with Histofix®-Safe?
Histofix®-Safe Is used for fixing histological and pathological specimens in a formalin zero-contact device for processing and preparation of biopsies for microscopic analysis.What is Histofix®-Safe?
Histofix®-Safe is a closed container used in pathology and histology that allows biological samples or biopsies to be fixed in a super-secure manner, avoiding contact with carcinogenic formaldehyde as much as possible.Which liquid is used in Histofix®-Safe?
Histofix®-Safe contains 4% diluted formalin, buffered and stabilized with methanol ready to fix biological samples or biopsies for microscopic analysis.What is Histofix®-Safe made of?
The Histofix®-Safe device is made of 100% recyclable plastics such as polypropylene, allowing for environmentally friendly sample disposal and recoverable plastic.How does the Histofix®-Safe cap work?
The Histofix®-Safe cap is bi-directional allowing formalin to flow from one container to the other as long as pressure is exerted on one of the containers. This is achieved by pressing the upper container until all the liquid flows into the lower container through the bidirectional valve. The sample will be immersed in the formaldehyde, starting the fixation process. If there is no pressure applied, formalin does not flow from one container to the other.Is Histofix®-Safe patented?
Yes, the Histofix Safe device is patented since January 2021 (Patent number WO2021/016060A9).Where can I buy Histofix®-Safe?
Histofix®-Safe can be purchased through our network of distributors globally.What is the advantage of Histofix®-Safe device?
The main advantage of Histofix®-Safe device is that biological samples or biopsies can be fixed and transported without the risk of inhaling or coming into contact with formalin vapor. The Histofix®-Safe device is also easy to store and transport, made of recyclable plastic, easy to use, intuitive.Which samples can I use for Histofix®-Safe?
Any type of biological or biopsy sample that is appropriate in terms of volume can be used. The sample should not be too large because it must fit inside and there must be enough formalin for appropriate fixation. In addition, the sample or biopsy should not be too small so that it cannot leak through the grid.Which volume should I choose?
The Histofix®-Safe device is available in three pack sizes: 20 mL, 30 mL and 40 mL. The capacity to be chosen depends on the volume of the biological sample. The volume of fixative should be proportional to the volume of the sample. In small biopsies, a volume of liquid 10 times the volume of tissue or more can usually be used.What is meant by fixation time?
The appropriate fixation time depends also on the volume of tissue and the type of specimen. In general, it can be between 6 hours for needle or endoscopic biopsies and 24 hours for surgical specimens.Is the Histofix®-Safe bearing the CE IVD mark?
Yes, the Histofix®-Safe device bears the CE IVD mark in compliance with the new regulation (EU) 2017/746.Literature
[1] SEAP-IAP, DOCUMENTO DE RECOMENDACIONES DE LA SOCIEDAD ESPAÑOLA DE ANATOMÍA PATOLÓGICA-IAP REFERENTE A LAS MEDIDAS DE SEGURIDAD ACONSEJADAS EN EL MANEJO DEL FORMALDEHÍD0 Y AL USO DE FIJADORES ALTERNATIVOS, Ángel Concha López, Ramiro Álvarez Alegret, Fina Autonell Reixach, Rocío Cabrera Pérez, Miguel Ángel Carrasco García, Ignacio Claros González, Sagrario García Sánchez , Javier Gómez Román, Teresa Hermida Romero, Teresa Hernández Iglesias, Antonio Martínez Lorente, Antonio Martínez Pozo, Beatriz Torio Sánchez, Enrique de Álava Casado.[2] Patent documentation: WO2021/016060A9 28 January 2021, EP3766579 – Container for biological Samples, EP 3 766 579 A1 EUROPEAN PATENT APPLICATION, WO2021016060 – Container for biological samples







