Packs sizes (1)
| code | packaging size | price per unit | box price per unit | |
|---|---|---|---|---|
| Code & packaging | Price per piece | |||

|
code
121076.1211
|
packaging size
1000 ml
|
price per unit
single
53,80€
|
box price per unit
45,73€x 6 units
|
Technical data
- Melting Point:
- -26 °C
- Boiling Point:
- 107 °C
- Density:
- 1.10 kg/l
- Physical Description:
- liquid
- Product Code:
- 121076
- Product Name:
- Hydrogen Peroxide 30% w/v (100 vol.) for analysis
- Quality Name:
- for analysis
- Specifications:
- Minimum assay (Perm.) w/v: 30.0%
Assay (in vol. O2) (Perm.): 100 vol.
Maximum limit of impurities
APHA colour: 10
Acidity: 0.0008 meq/g
Non-volatile matter: 0.005 %
Chloride (Cl): 0.0001%
Nitrogen compounds (as N): 0.001%
Phosphate (PO4): 0.0005 %
Sulfate (SO4): 0.0005%
Heavy metals (as Pb): 0.0001%
Metals by ICP [in mg/Kg (ppm)]
Ag: 0.05
Al: 0.2
As: 0.5
Au: 0.1
B: 0.5
Ba: 0.1
Be: 0.02
Bi: 0.05
Ca: 0.5
Cd: 0.05
Co: 0.02
Cr: 0.02
Cu: 0.02
Fe: 0.1
Ga: 0.05
Ge: 0.05
Hg: 0.1
In: 0.05
K: 5
Li: 0.02
Mg: 0.1
Mn: 0.02
Mo: 0.02
Na: 10
Ni: 0.05
Pb: 0.1
Pt: 0.1
Sb: 0.02
Sr: 0.02
Ti: 0.05
Tl: 0.02
V: 0.02
Zn: 0.1
Zr: 0.05
- Hazard pictograms
-
- UN:
- 2014
- Class/PG:
- 5.1(8)/II
- ADR:
- 5.1(8)/II
- IMDG:
- 5.1(8)/II
- IATA:
- 5.1(8)/II
- WGK:
- 1
- Storage:
- Room Temperature.
- Signal Word:
- Danger
- GHS Symbols:
- GHS07
GHS05
- H Phrases:
- H302
H318
H332
- P Phrases:
- P264
P270
P280
P301+P312
P305+P351+P338
P310
P330
P501
- Master Name:
- Hydrogen Peroxide 30% w/v *(100 vol)
- Synonyms Long Text:
- Hydrogen Dioxide, Hydroperoxide
- EINECS:
- 231-765-0
- CS:
- 28470000
- Index Nr.:
- 008-003-00-9
Documents
Inquiry
Comments
Hydrogen peroxide (H2O2), also known as hydrogen peroxide, dioxogen, dioxidane or dihydrogen peroxide is a chemical compound with characteristics of a highly polar, strongly hydrogen-bonded liquid, such as water, usually of slightly more viscous liquid appearance. It is known to be a powerful oxidizer.At room temperature it is a colorless liquid with a pungent, unpleasant odor. Small amounts of gaseous hydrogen peroxide occur naturally in the air. Hydrogen peroxide is very unstable. It decomposes slowly into oxygen and water, releasing a large amount of heat. Its decomposition rate can be greatly increased in the presence of catalysts.
Although it is not flammable, it is a powerful oxidizing agent which, when it comes into contact with organic matter or some metals such as copper, silver or bronze, can cause spontaneous combustion.
Hydrogen peroxide is found in low concentrations (3 to 9 %) in many household products for medicinal uses and as a clothing and hair bleach. In industry it is used in higher concentrations, for bleaching fabrics and paper pulp, and at 90% as a component of rocket fuels and for making foam rubber and organic chemicals. In other areas, such as research, it is used to measure the activity of some enzymes, such as catalase.
Physical and chemical properties
Pure hydrogen peroxide (H2O2) is a dense, clear liquid, with a density of 1.47 g/cm³ at 0 °C. Its melting point is -0.4 °C. Its normal boiling point is 150 °C.
Stereochemistry
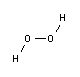
Similar to water, hydrogen peroxide has an axis of symmetry (axis rotated 180°). It has three conformations: cis-planar (symmetry group C2v), cis-non-planar (symmetry group C2) and trans-planar (symmetry group C2h).
Reactivity
Concentrated hydrogen peroxide is a dangerously reactive substance, because its decomposition to generate water and oxygen is highly exothermic. The following thermochemical reaction demonstrates that fact:
2 H2O2 (l) → 2 H2O (l) + O2 (g) ΔHº = -98.2 kJ/mol.
Committed as oxidizing and reducing agent.
Hydrogen peroxide is capable of acting either as an oxidizing agent or as a reducing agent. The equations below present the half-reactions in acidic media:
2 H+ (aq) + H2O2 (aq) + 2 e- → 2 H2O (l) Eo - net = 1.77 V
O2 (g) + 2 H+ + 2 e- → H2O2 (aq) Eo - lattice = 0.695 V2
In basic solution, the potentials corresponding to the standard electrode are 0.87 V (volts̠) for the reduction of hydrogen peroxide and 0.08 V for its oxidation.
Production
Formerly hydrogen peroxide was prepared by electrolysis of an aqueous solution of sulfuric acid or acid ammonium bisulfate (NH4HSO4), followed by hydrolysis of peroxodisulfate ((SO4)2). Hydrogen peroxide is now obtained almost exclusively by the autooxidation of a 2-alkoxy-anthrahydroquinone (or 2-alko-9-10-dihydroxyanthracene) to the corresponding 2-alkoanthraquinone in a method called the "anthraquinone process".
In 1994, world H2O2 production was 1.9 million tons. In 2006 it grew to 2.2 million, most of it with a concentration of 70 % or less. In that year, a kilogram of hydrogen peroxide was selling for US$1.5.
Discovery
Hydrogen peroxide was first described in 1818 by Louis Jacques Thénard, who produced it by treating barium peroxide with nitric acid. An improved version of this process uses hydrochloric acid, followed by the addition of sulfuric acid to precipitate the barium sulfate byproduct. The Thénard process was used from the end of the 19th century until the middle of the 20th century.
It was long believed that pure hydrogen peroxide would be unstable, since all early attempts to separate it from water, which is present during synthesis, failed. This instability was due to trace impurities (transition metal salts), which catalyze the decomposition of this peroxide. Pure hydrogen peroxide was first obtained in 1894 - almost 80 years after its discovery - by Richard Wolffenstein, who produced it by vacuum distillation.
Determining the molecular structure of hydrogen peroxide was very difficult. In 1892, the Italian physico-chemist Giacomo Carrara (1864-1925) determined its molecular mass by cryoscopic descent, which confirmed its molecular formula to be H2O2. At least half a dozen hypothetical molecular structures appeared to be consistent with the available evidence. In 1934, the English mathematical physicist William Penney and the Scottish physicist Gordon Sutherland proposed a molecular structure very similar to the one accepted today.
Applications
Industrial
Hydrogen peroxide has many industrial uses, such as:
- Bleaching of fabrics, cotton and paper pulp.
- It is increasingly used as a substitute for chlorine.
- In the food industry it is widely used to bleach cheese, chicken, meat, bones, as well as for the production of vegetable oils.
- In the chemical industry it is used as a reagent.
- It is very important in the manufacture of pharmaceuticals.
- It is also being used for dental bleaching. Industrial hydrogen peroxide usually has concentrations of more than 30 %, unlike the one for domestic use bought in pharmacies and supermarkets, which usually contains only 3 %.
Aerospace
Hydrogen peroxide is used in the aerospace industry as a fuel in monopropellant rocket engines or for oxygen supply in bipropellant engines. This peroxide is generally used at a concentration of 90%. It is extremely explosive.
Artistic
Hydrogen peroxide is used in restoration work. In many old paintings, white pigments based on lead(II) carbonate have discolored due to the formation of lead(II) sulfide, which is particularly black. Hydrogen peroxide reacts in such a way as to convert lead(II) sulfide into lead(II) sulfate (white color). Both salts are insoluble in water. The reaction is as shown in the following equation.
PbS (s) + 4 H2O2 (aq) → PbSO4 (s) + 4 H2O (l)
Therapeutic use
Generally, major health agencies around the world recognize hydrogen peroxide dilutions up to 6 % as safe for use as an antimicrobial agent, oxidizing agent and other purposes. Because of its oxidizing effect, it has been used as an antiseptic and antibacterial agent for many years. Its use has declined in recent years due to the popularity of other substitute products. It is still used in many hospitals, medical centers and clinics.
Disinfection
Hydrogen peroxide is a general antiseptic. Its mechanism of action is due to its oxidizing effects: it produces hydroxyl (OH-) and free radicals that attack a wide variety of organic compounds, including lipids and proteins that make up the cell membranes of microorganisms. The enzyme catalase present in the tissues rapidly degrades hydrogen peroxide, producing oxygen, which hinders the germination of anaerobic spores.
It is used in dermo-applications, denture cleaning and oral disinfection, as well as, in the field of optics, in contact lens disinfection.
In addition, taking advantage of the peroxidase activity present in blood, it is also used together with phenolphthalein to detect the presence of blood (Kastle-Meyer test).
Synonyms of Hydrogen Peroxide: Dioxidane; Oxidanyl; Perhydroxic acid; 0-hydroxyol; Dihydrogen dioxide; Oxygenated water; Peroxaan
FAQs
What are synonyms for Hydrogen Peroxide?
Synonyms for Hydrogen Peroxide are Dioxidane, Oxidanyl; Perhydroxic Acid; 0-Hydroxyol, Dihydrogen dioxide, Oxygenated water, Peroxaan and H2O2.What is the CAS number of Hydrogen Peroxide?
The CAS number of Hydrogen Peroxide is 7722-84-1.
CAS Hydrogen Peroxide?
The CAS number of Hydrogen Peroxide is 7722-84-1.
CAS 7722-84-1?
The CAS number 7722-84-1 is assigned to Hydrogen Peroxide.



