Packs sizes (3)
| code | packaging size | price per unit | box price per unit | |
|---|---|---|---|---|
| Code & packaging | Price per piece | |||

|
code
141621.1211
|
packaging size
1000 g
|
price per unit
single
$72,30
|
box price per unit
$61,46x 6 units
|

|
code
141621.1214
|
packaging size
5 kg
|
price per unit
single
$214,80
|
box price per unit
$182,58x 4 units
|

|
code
141621.0416
|
packaging size
25 kg
|
price per unit
single
Request a quote
|
box price per unit
|
Technical data
- Melting Point:
- 186 °C
- Solubility:
- water 1,970 g/l at 15 °C
- Physical Description:
- solid
- Product Code:
- 141621
- Product Name:
- D(+)-Sucrose (USP-NF, BP, Ph. Eur., JP) pure, pharma grade
- Quality Name:
- pure, pharma grade
- Specifications:
- Identity according to Pharmacopoeias:: passes test
Specific rotation α 25/D c=26 (in H2O): > +65.9°
Specific rotation α 20/D c=26 (in H2O): +66.3 - + 67.0°
Maximum limit of impurities
Appearance of solution: passes test
Acidity or alkalinity: passes test
Loss on drying at 105°C: 0.1%
Residue on ignition (as SO4): 0.02 %
Sugars Reducing: passes test
Chloride (Cl): 0.0035%
Sulfate (SO4): 0.006%
Sulfite (SO2): 0.0010%
Specific conductance at 20°C: 35x10-6 ohm-1 cm-1
Inverted sugar: passes test
Colour value: 45
Residual solvents (Ph.Eur/USP): passes test
Dextrin: passes test
Heavy metals (as Pb): 0.0005%
Ba: passes test
Ca: passes test
Fe: 0.0005 %
- WGK:
- nwg
- Storage:
- Room Temperature.
- Master Name:
- Saccharose
- Synonyms Long Text:
- b-D-Fructofuranosyl-a-D-Glucopyranoside, Sucrose, Sugar
- EINECS:
- 200-334-9
- CS:
- 17019990
Documents
Inquiry
Comments
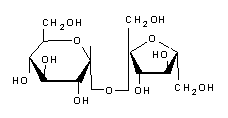
Synonyms: Sugar, Saccharose, α-D-glucopyranosyl-(1→2)-β-D-fructofuranoside, β-D-fructofuranosyl-(2→1)-α-D-glucopyranoside, β-(2S,3S,4S,5R)-fructofuranosyl-α-(1R,2R,3S,4S,5R)-glucopyranoside, α-(1R,2R,3S,4S,5R)-glucopyranosyl-β-(2S,3S,4S,5R)-fructofuranoside, Dodecacarbon monodecahydrate, ((2R,3R,4S,5S,6R)-2-[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxapent-2-yl]oxy-6-(hydroxymethyl)oxahexane-3,4,5-triol)Sucrose or saccarose is a sugar with a sweet taste. It is extracted from certain plants, mainly sugar cane and sugar beet, and is widely used in human nutrition. It is a diholoside composed of glucose and fructose, whose normalized name is α-D-glucopyranosyl-(1↔2)-β-D-fructofuranoside, usually abbreviated as Glc-Fru. In English, it is referred to as sucrose, hence the abbreviation Suc for this compound sometimes encountered in the literature. - Carbohydrates, especially sucrose, used to be called carbohydrates because of an old experiment on the dehydrogenation of white sugar with concentrated sulfuric acid, in which sucrose with the molecular formula C12H22O11 was decomposed into water H2O and carbon black. From this it had been erroneously deduced that the formula for sucrose could be written (H2O)11C12, which would correspond to a carbohydrate. The term was made obsolete by the work of Walter Norman Haworth, who established the structure of carbohydrates in the early 20th century, and has since fallen out of use. In the English-speaking world, however, the term is still in common use. - Properties - Structure - The sucrose molecule is a diholoside consisting of a glucose residue and a fructose residue joined by an α(1↔2)β osidic bond. Its molecular formula is C12H22O11 and its molar mass is 342.3 g mol-1. Unlike most other diholosides, sucrose is formed by linking two oses at their reducing ends, which makes it a non-reducing sugar. In addition, only the α-anomer of glucopyranose and the β-anomer of fructofuranose form sucrose. - Sucrose crystallizes in the monoclinic crystal system according to space group P21 with crystal parameters a = 1.086 31 nm, b = 0.870 44 nm, c = 0.776 24 nm and β = 102.938° - The purity of sucrose is measured polarimetrically. More specifically, the rotation of the plane of polarization of polarized light passing through an aqueous sugar solution is measured. The specific rotation (en) at 20 °C, measured using the sodium D-line at 589 nm, is +66.47°. Commercial sucrose is controlled in this way. It does not deteriorate under normal conditions. - Sucrose has five natural isomers that differ in the position of the osidic bond: Trehalulose ⇒ glucose α(1→1) fructose ; Turanose ⇒ glucose α(1→3) fructose (reducing diholoside) ; Maltulose ⇒ glucose α(1→4) fructose ; Leucrose ⇒ glucose α(1→5) fructose (reducing diholoside) ; isomaltulose (palatinose) ⇒ glucose α(1→6) fructose (reducing diholoside). - Reactions involving sucrose-Various chemical transformations involving sucrose as a reactant are conceivable: -Thermal decomposition-The thermal decomposition of sucrose can be represented as a two-step reaction: -first, dehydration (by sulfuric acid H2SO4), producing water and soot: - C12H22O11 → 12 C + 11 H2O ; -and then oxidation (with oxygen from the air) of carbon to carbon dioxide : - 12 C + 12 O2 → 12 CO2. - Caramelization - sucrose does not melt when heated, but decomposes to caramel at about 186 °C. - Combustion - Sucrose burns in an exothermic reaction to form water and carbon dioxide, as all carbohydrates do. - With chloric acid - With chloric acid HClO3, sucrose releases water, carbon dioxide, and hydrogen chloride (HCl) : - 8 HClO3 + C12H22O11 → 11 H2O + 12 CO2 + 8 HCl. - Chemical properties - Sucrose is a non-reducing sugar , the hemiacetal carbon of glucose and the hemiacetal carbon of fructose are involved in the osideic bond. It is not hygroscopic and cannot undergo mutarotation. - Hydrolysis - Hydrolysis of sucrose causes the osidic bond to be cleaved, releasing glucose and fructose in equimolar amounts. However, this reaction is so slow that an aqueous sucrose solution can remain practically stable for years. In contrast, it proceeds much faster in the presence of a saccharase (sucrose isomaltase, invertase, sucrose alpha-glucosidase) or an acid such as potassium bitartrate HOOC-CHOH-CHOH-COOK or lemon juice, which are weak acids. The same happens in gastric juice, which participates in digestion by hydrolyzing carbohydrates such as sucrose. - The action of invertase on sucrose produces invert sugar, which consists of equal amounts of glucose and fructose. - Biosynthesis of sucrose - The biosynthesis of sucrose is carried out by sucrose phosphate synthase from UDP-glucose and fructose-6-phosphate. The energy required for this reaction comes from the cleavage of UDP. Sucrose is produced by plants and cyanobacteria, but not by other living organisms. It occurs naturally, along with fructose, in many plants consumed in the human diet. It is the main carbohydrate of many fruits such as pineapples and apricots; other fruits such as pears and grapes have fructose as their main carbohydrate. - The complete synthesis of sucrose was achieved by the Canadian chemist Raymond Lemieux in 1953. - Physical properties - Sucrose caramelizes at 160 °C. - Sucrose is highly soluble in water. The solubility of sucrose in non-aqueous solvents is generally lower. In addition, sucrose is not soluble in nonpolar solvents. High values are obtained with condensed ammonia (72%), dimethyl sulfoxide (42%) and methylamine (> 25%). Lower values are obtained with liquid sulfur dioxide, formic and acetic acids, dimethylformamide, pyridine (6%), propylene glycol, glycerol (7%), methanol, ethanol, acetone and dioxane. - The sugar content is indicated by the Brix degree (which corresponds to the mass percentage at 20 °C). The concentration of an aqueous solution can be determined by measuring the density with a mustimeter or the refractive index with a refractometer. - Sweetening Properties - The sweetening power of sucrose serves as a reference value in the sweetener scale, i.e., it is conventionally taken as 1. The average detection limit of sucrose is 0.017 mol l-1 (5.82 g/liter). - Sources of sucrose - In nature, sucrose is found in a wide variety of plants, especially in their roots, fruits, and nectar, where it is used to store energy from photosynthesis. Many mammals, birds, insects and bacteria feed on the sucrose in plants, some even as their main food. Bees play a special role in human nutrition in this regard, as they produce honey from nectar, which is consumed in all parts of the world. However, the sugars in honey are predominantly glucose and fructose, with only traces of sucrose. As fruits ripen, the sucrose content usually increases very rapidly, but there are also fruits that contain almost no sucrose. Examples include grapes, cherries, blueberries, blackberries, figs, pomegranates, tomatoes, avocados, lemons and limes. - Uses in the Food Industry - Sugar as an Important Food Ingredient - Sucrose is one of the most commonly used ingredients in the food industry. It forms the basis for certain industries such as confectionery, bakery, jam and beverage. - In the pharmaceutical industry, it is used as an excipient. - Physical Chemistry - Sucrose is highly soluble in water, with solubility increasing with temperature. It is relatively stable, but can ferment (a generally undesirable development except in alcoholic beverages) or hydrolyze into glucose and fructose (in confectionery language: invert), although this phenomenon can be controlled. - When used in the beverage industry, sucrose can invert by itself during heat treatment. In addition, since invert sugar is more soluble than sucrose, it can act as a water binder in its presence and prevent sucrose from crystallizing. - Sucrose reduces the water activity of products that contain a lot of sucrose, allowing them to be preserved. - It can also be used as a texture aid due to its agglomerating properties. - Grain size - Different crystal sizes according to the needs of the industry : granulated sugar; sugar couleur; powdered sugar; granulated sugar. - Use in fermentation - Sucrose is one of the most classic fermentation substrates. Many microorganisms can ferment it, including the highly evolved Saccharomyces cerevisiae. - Uses in Agriculture - Sucrose is used in agriculture. Spraying very small doses of soluble sugars in orchards has an insect repellent effect by altering the perception of the plant by pests.




