Packs sizes (9)
| code | packaging size | price per unit | box price per unit | |
|---|---|---|---|---|
| Code & packaging | Price per piece | |||

|
code
221881.1611
|
packaging size
1000 ml
|
price per unit
single
93,10€
|
box price per unit
79,13€x 6 units
|

|
code
221881.1612
|
packaging size
2.5 l
|
price per unit
single
199,00€
|
box price per unit
169,15€x 4 units
|

|
code
221881.16153
|
packaging size
4 l
|
price per unit
single
327,80€
|
box price per unit
278,63€x 4 units
|

|
code
221881.0314
|
packaging size
5 l
|
price per unit
single
376,60€
|
box price per unit
320,11€x 4 units
|

|
code
221881.0515
|
packaging size
10 l
|
price per unit
single
Request a quote
|
box price per unit
|

|
code
221881.0316
|
packaging size
25 l
|
Product discontinued. Alternative 221881.0314 (please check specifications).
|
|

|
code
221881.0537
|
packaging size
30 l
|
price per unit
single
Request a quote
|
box price per unit
|

|
code
221881.0519
|
packaging size
200 l
|
price per unit
single
Request a quote
|
box price per unit
|

|
code
221881.0574
|
packaging size
1000 l
|
price per unit
single
Request a quote
|
box price per unit
|
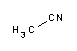
Technical data
- Melting Point:
- -48 °C
- Boiling Point:
- 80 - 82 °C
- Density:
- 0.781 kg/l
- Refractive Index:
- 20/D 1.344
- Physical Description:
- liquid
- Product Code:
- 221881
- Product Name:
- Acetonitrile (Reag. Ph. Eur.) for HPLC gradient / UHPLC supergradient grade, ACS
- Quality Name:
- for HPLC gradient / UHPLC supergradient grade, ACS
- Specifications:
- Minimum assay (G.C.): 99.9%
Identity: IR passes test
Density 20/4: 0.779-0.783
Suitability: for gradient acc. to ACS: passes test
Range of Distillation (>95% dist.): 80-82°C
Maximum limit of impurities
APHA colour: 10
Acidity: 0.0005 meq/g
Alkalinity: 0.0001 meq/g
Non-volatile matter: 0.0001 %
Base line drift (210 nm): 10 mUA
Water (H2O): 0.015 %
Gradient at 210 nm: 1 mUA
Gradient at 254 nm: 0.5 mUA
Fluorescence at 254 nm (as quinine): 1 ppb
Fluorescence at 365 nm (as quinine): 0.5 ppb
UV Spectrum (1cm cell; Ref.: water):
Transmittance at 190 (Cut off) nm: ≥30%
Transmittance at 193 nm: ≥60%
Transmittance at 195 nm: ≥80%
Transmittance at 200 nm: ≥90%
Transmittance at 230-400 nm: ≥98%
Data of interest in HPLC:
Rohrschneider Polarity: 5.8
Eluotropic value e° (Al2O3): 0.65
Sol. H2O in solv. at 20°C: miscible
Microfiltered product (0.2 μm) and bottled under nitrogen atmosphere.
Conforms to Acetonitrile for chromatography and Acetonitrile R1 according to Reag. Ph. Eur.
- Hazard pictograms
-
- UN:
- 1648
- Class/PG:
- 3/II
- ADR:
- 3/II
- IMDG:
- 3/II
- IATA:
- 3/II
- WGK:
- 2
- Storage:
- Room Temperature.
- Signal Word:
- Danger
- GHS Symbols:
- GHS02
GHS07
- H Phrases:
- H225
H332
H312
H302
H319
- P Phrases:
- P210
P233
P240
P241
P242
P243
P261
P270
P271
P280
P301+P330+P331
P303+P361+P353
P304+P340
P305+P351+P338
P312
P322
P363
P370+P378
P501
P337+P313
- Master Name:
- Acetonitrile
- Synonyms Long Text:
- Cyanomethane, Ethanenitrile, Methyl Cyanide
- EINECS:
- 200-835-2
- CS:
- 2926 90 70 90
- Index Nr.:
- 608-001-00-3









