Packs sizes (3)
| code | packaging size | price per unit | box price per unit | |
|---|---|---|---|---|
| Code & packaging | Price per piece | |||

|
code
A3928,0500PE
|
packaging size
500 ml
|
price per unit
single
44,80€
|
box price per unit
|

|
code
A3928,1000GL
|
packaging size
1 L
|
price per unit
single
52,50€
|
box price per unit
|

|
code
A3928,2500PE
|
packaging size
2.5 L
|
price per unit
single
92,70€
|
box price per unit
|
Technical data
- Refractive Index:
- n20/D 1.377 (abs.)
- Physical Description:
- Liquid
- Product Code:
- A3928
- Product Name:
- 2-Propanol for molecular biology
- Specifications:
- DNases/RNases/Proteases: not detectable
Assay (GC): min. 99.8 %
Acidity/Alkalinity: max. 0.0005 meq/g
Non-volatile matter: max. 0.0005 %
Total P: max. 0.00005 %
Total S: max. 0.00005 %
Ethanol: max. 0.01 %
Methanol: max. 0.1 %
n-Propanol: max. 0.05 %
Water (K.F.): max. 0.1 %
Ca: max. 0.00002 %
Cu: max. 0.000002 %
Fe: max. 0.00001 %
Mg: max. 0.00001 %
Pb: max. 0.000002 %
Zn: max. 0.00001 %
- Hazard pictograms
-
- UN:
- 1219
- Class/PG:
- 3/II
- ADR:
- 3/II
- IMDG:
- 3/II
- IATA:
- 3/II
- WGK:
- 1
- Storage:
- RT
- Signal Word:
- Danger
- GHS Symbols:
- GHS02
GHS07
- H Phrases:
- H225
H319
H336
- P Phrases:
- P210
P280
P303+P361+P353
P305+P351+P338
P405
P501
- EINECS:
- 200-661-7
- CS:
- 29051200
- Index Nr.:
- 603-117-00-0
Documents
Inquiry
Comments
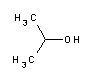
2-Propanol (IUPAC name propan-2-ol), also known as isopropyl alcohol or isopropanol (abbreviated IPA), is the simplest non-cyclic secondary alcohol and a monohydric alcohol.Occurrence
Naturally, 2-propanol occurs in apples (Malus domestica) and geraniums (Pelargonium graveolens).
Production and synthesis
Large-scale production of 2-propanol is achieved by hydration of propene over acidic ion exchange resins as a catalyst:
CH3=CH-CH3 + H2O → CH3-CHOH-CH3 (catalyzed by acidic ion exchange resins)
Alternatively, 2-propanol can be obtained by catalytic hydrogenation of acetone:
CH3-CO-CH3 + H2 → CH3-CHOH-CH3 (catalyzed)
By reversing the second reaction, acetone is produced on a large scale from isopropanol by oxydehydrogenation, i.e. dehydrogenation with simultaneous oxidation of the resulting hydrogen with oxygen to water.
Properties
Physical properties
Isopropanol is a colorless, highly volatile and flammable liquid that has a slightly sweet, pungent odor (this is characteristic and reminiscent of hospitals and doctors' offices, as isopropanol is a component of many disinfectants). At -88 °C, the liquid solidifies to a colorless solid. The boiling point under normal pressure is 82 °C. Isopropanol is homogeneously miscible with water in any ratio and forms a constant boiling (azeotropic) mixture at 80.4 °C and 12.1 % water content. The compound forms azeotropic boiling mixtures with a number of other solvents. No azeotropes are formed with methanol, ethanol, 1-propanol, n-butanol, iso-butanol, sec-butanol, cyclohexanol, ethanediol, ethylbenzene, acetone, diethyl ether, 1,4-dioxane, methyl acetate and dimethylformamide.
Thermodynamic properties
The vapor pressure curve is obtained according to Antoine corresponding to log10(P) = A−(B/(T+C)) (P in bar, T in K) with A = 4.57795, B = 1221.423 and C = −87.474 in the temperature range from 359.0 to 508.24 K.
Compilation of the most important thermodynamic properties
Standard enthalpy of formation: ΔfH0liquid = −318.2 kJ·mol−1 ; ΔfH0gas = −272.3 kJ·mol−1
Standard entropy: S0liquid = 180.58 J·mol−1·K−1 (as liquid)
Combustion enthalpy: ΔcH0liquid = −2005.8 kJ·mol−1
Heat capacity:
Cp (liquid) = 161.2 J·mol−1·K−1 (25 °C) or 2,68 J·g−1·K−1 (25 °C)
Cp (gas) = 89.32 J·mol−1·K−1 (25 °C) or 1.49 J·g−1·K−1 (25 °C)
Critical temperature: Tc = 508.3 K
Critical pressure: pc = 47.6 bar
Acentric factor: ωc = 0.66687
Enthalpy of fusion (at melting point): ΔfH0 = 5.41 kJ·mol−1
Enthalpy of vaporization (at normal pressure boiling point): ΔVH0 = 39.85 kJ·mol−1
The temperature dependence of the enthalpy of vaporization can be calculated according to the equation:
ΔVH0=A·e(−αTr)(1−Tr)β (ΔVH0 in kJ/mol, Tr =(T/Tc) reduced temperature) with A = 53.38 kJ/mol, α = −0.708, β = 0.6538 and Tc = 508,3 K in the temperature range between 298 K and 380 K.
Safety parameters
2-Propanol forms highly flammable vapor-air mixtures. The compound has a flash point at 12 °C. The explosion range is between 2 vol% (50 g/m3) as the lower explosive limit (LEL) and 13.4 vol% (335 g/m3) as the upper explosive limit (UEL). A correlation of the explosive limits with the vapor pressure function results in a lower explosive point of 10 °C as well as an upper explosive point of 39 °C. The explosive limits are temperature and pressure dependent. Increased temperatures lead to an expansion of the explosive range. A reduction in pressure leads to a reduction in the explosive range. The lower explosive limit changes little up to a pressure of 100 mbar and increases only at pressures lower than 100 mbar. The upper explosive limit decreases analogously with decreasing pressure. The maximum explosive pressure at 50 °C is 8.6 bar. With increasing temperature and decreasing initial pressure, the maximum explosive pressure decreases.
The limiting oxygen concentration is 8.7 % by volume at 20 °C, and 8.1 % by volume at 100 °C. It changes only slightly under reduced pressure. The maximum experimental safe gap (MESG) was determined to be 0.99 mm. This results in an assignment to explosion group IIA. The ignition temperature is 425 °C. The substance thus falls into temperature class T2. The electrical conductivity is rather low at 5.8·10-6 S·m-1. In aqueous solutions, the flash point of <20 °C changes only slightly up to a water content of 80 mol%.
Chemical properties (safety)
2-Propanol, like other secondary alcohols, can form explosive peroxides with atmospheric oxygen. For example, a peroxide content of 1% was found in containers in which isopropanol had been stored for ten years; peroxide concentrations of up to 4.2% were reported. As a result, accidents, some serious, have occurred when distilling off 2-propanol to dryness. It is therefore advisable to test isopropanol for peroxides before distilling off.
Toxicology
The vapors have an anesthetic effect. Contact causes irritation of the eyes and mucous membranes. Adequate ventilation should be provided when handling. No evidence of sensitizing or mutagenic properties was found in animal studies.
The lethal dose is 5.05 g·kg−1 in rats (LD50 oral) and 12.8 g·kg−1 in rabbits (LD50 dermal).
Uses
• Solvent for fats, resins, varnishes, ink.
• Extraction and purification of natural products
• Solvent for crystallization and purification of organic substances
• Precipitation of nucleic acids
• Cleaning agent (fat solvent) in industry and household (for example in eyeglass cleaning wipes)
• Solvent and diluent in cosmetic and pharmaceutical preparations.
• Additive to antifreeze in the cooling system or in the windshield washer system in cars and trucks
• component of door lock and car window deicers
• Ingredient of so-called fuel system cleaners, which are added to the fuel of motor vehicles to dissolve residues and water in the system
• Additive in offset printing presses with alcohol dampening systems to reduce the surface tension of the dampening solution (so-called "wiping water additive")
• Production of disinfectants (acts as bactericide, tuberculocide, fungicide and limited virucide)
• Defoaming agent
• Reactant in the Meerwein-Ponndorf-Verley reduction of aldehydes or ketones
• Production of isopropylamine
• Reactant in the synthesis of sarin
• For wet playing of records: 50 % isopropanol mixed with 50 % distilled water
• For removing grease, lubricant and silicone residues during paint preparation on vehicles. Mixture diluted with up to 50 percent water.
• For cleaning optical surfaces (objectives and eyepieces), especially in microscopy: 15 % isopropanol with 85 % n-hexane
• Cleaning of soldered boards, removal of flux residues (only alcohol-based fluxes)
• Removal of the sticky residue (after curing under UV light) during nail modeling
• In hydraulic fracturing, it serves as a corrosion inhibitor in the frac fluids used
• As an alcohol component in fog chambers
• Preservation of wet preparations
Isopropanol is used for the precipitation of nucleic acids. Whereas with ethanol the amount required is 2.5 times the volume of the sample containing nucleic acids, isopropanol is added in an equal volume. Precipitate forms even without cooling. The advantage of isopropanol is the reduction of the total volume. However, the disadvantages are that isopropanol cannot be removed as well as ethanol by lyophilization, and salts can co-precipitate with DNA. Therefore, precipitation with isopropanol is performed after precipitation with ethanol.







