Biochemical processes are markedly impaired by even small changes in the concentrations of free H+ ions.
It is therefore usually required to stabilise the H+ concentration in vitro without affecting the system's functioning. A buffer keeps the pH of a solution constant by taking up protons that are released during reactions, or by releasing protons when they are consumed by reactions. The observation that partially neutralised solutions of weak acids or bases are resistant to changes in pH when small amounts of strong acids or bases are added led to the concept of the ‘buffer’. In the table, the most common biological buffers are listed according to their optimal buffer range. The table also gives some hints for potential applications and shows advantages or disadvantages of the listed buffer substances.
Buffer systems described in the literature are usually used for experiments to enable direct comparison of results.
Again and again, it is shown that the conditions in experiments – even in standard systems – could be optimised. In our InfoPoint "Biological Buffers" we provide a selection of important information from the literature that might help you in solving your everyday problems as well as give recommendations for the development and optimization of your test systems: Which requirements do biological buffers have to fulfil? Which criteria need to be taken into account? How is the pH value adjusted effectively?
Biological buffers - survey
| Prod. No. | Description | pKa | useful pH range | dpKa/dT# | Comment |
| (100mM, 25°C) | |||||
| A1067 | Glycine 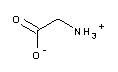 |
pK1=2.35 | 2.2 - 3.6 | Component of the Laemmli buffer (A1415) for SDS-PAGE of proteins; interferes with protein assays according to Lowry (>1mM) and Bradford (>100mM); available in different grades, e.g. in "molecular biology grade" | |
| A1045 | 4.76 | 3.7 - 5.6 | 0.002 | Sodium salt of acetic acid (e.g. A3701, acetic acid 100%). Often used together with ethanol for precipitation of nucleic acids. | |
| Acetate | We offer a selection of different grades of sodium, potassium and magnesium; many of these products are available in solution as well. | ||||
| 131457 | 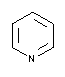 |
5.23 | 4.9 - 5.9 | 0.014 | Do not autoclave! |
| Pyridine | |||||
| A1074 | MES | 6.10 | 5.5 - 6.7 | -0.011 | Zwitterionic "Good" buffer; substitute for cacodylic acid. Used in culture media for plant cells; not metabolized by bacteria and eukaryotic cells. Interferes with protein assay according to Lowry but not with BCA test. We also offer potassium salt, as well as alternative quality grades. |
| A1351 | Citrate | pK3=6.40 | 5.5 - 7.2 | 0.0 | tri-sodium salt of citric acid. Binds several proteins and forms complexes with metal ions; interferes with protein assays according to Lowry (>2.5mM), Bradford (>50mM) and with the BCA test (<1mM). |
| The pH range is set by mixing together citric acid and sodium citrate. Both products are available in multiple quality grades. | |||||
| A1025 | 6.46 | 5.8 - 7.2 | -0.017 | Important buffer for protein and nucleic acid systems. Used as a substitute for cacodylic acid buffer systems, in IEF and 2D-gel electrophoresis. Interferes with BCA-test. Also available in "molecular biology grade": A3992 | |
| Bis-Tris | |||||
| A1046 | Phosphate | pK2=7.20 | 5.8 - 8.0 | -0.0028 | di-sodium hydrogenphosphate; substrate/inhibitor of various enzymes; precipitates bivalent cations; pKa increases on dilution, but is only slightly depending on the temperature. |
| (Hydrogenphosphate) | PBS (phosphate-buffered saline, available as tablets, e.g. A9201) is widely used in biochemistry, microbiology, immunology, and cell biology. | ||||
| Interferes with BCA and Lowry assay at concentrations >250mM. Also available as di-ammonium or di-potassium salt (A1042) as well as in alternative grades. | |||||
| A1079 | PIPES | 6.76 | 6.1 - 7.5 | -0.0085 | Zwitterionic "Good" buffer. Interferes with protein assay according to Lowry. Can form radicals, not suitable for redox studies. |
| A1060 | ACES | 6.78 | 6.1 - 7.5 | -0.020 | Zwitterionic "Good" buffer; binds Cu2+-ions and interferes with Lowry assay. Significant absorption at 230 nm. |
| A1073 | Imidazole | 6.95 | 6.2 - 7.8 | -0.020 | 'His-tag' bound recombinant proteins are effectively purified by nickel-NTA-columns. The bound proteins can be eluted by imidazole, which competes with the his-tagged protein for the nickel ions. Furthermore, imidazole is used as buffer substance for enzymatic reactions or for DNA denaturation. A concentration of 1 M reduces the melting temperature of DNA by approx. 13°C and changes the mobility in the gel electrophoresis. |
| Caution: DEPC reacts with the non-protonated form of imidazole to N-Carbethoxyimidazole. | |||||
| The product is available in multiple quality grades, e.g. in "molecular biology grade", A1378. | |||||
| A1062 | 7.09 | 6.4 - 7.8 | -0.016 | Zwitterionic "Good" buffer; binds Cu2+-ions and interferes with Lowry assay. | |
| BES | |||||
| A1076 | MOPS | 7.14 | 6.5 - 7.9 | -0.011 | Zwitterionic "Good" buffer with low ion binding. Interferes with Lowry assay. |
| Also available in "molecular biology grade", (A2947). | |||||
| A1084 | 7.40 | 6.8 - 8.2 | -0.020 | Zwitterionic "Good" buffer. Interferes with protein assay according to Lowry, but not with the BCA test. | |
| TES | |||||
| A1069 | HEPES | 7.48 | 6.8 - 8.2 | -0.014 | Zwitterionic "Good" buffer; widely used buffer in biological studies. In cell culture media, it is employed as a substitute or supplement for the bicarbonate buffer. |
| Interferes with protein assay according to Lowry, but not with the BCA and the Bradford assay. HEPES does not bind metal ions, but it can form radicals and is not suitable for redox studies. The product is available in various quality grades as sterile 1M solution pH 7.5 (A6916) or pH 8 (A6906) respectively. | |||||
| A1072 | HEPPSO | 7.85 | 7.1 - 8.5 | -0.010 | Binds Cu2+-ions; interferes with the Lowry assay but is compatible with the BCA test. Can form radicals and is not suitable for redox studies. |
| A1085 | Tricine | 8.05 | 7.4 - 8.8 | -0.021 | Zwitterionic "Good" buffer; can replace Tris in many buffer systems. Exchange of tricine against glycine in buffer for SDS-PAGE improves the resolution in the range of 1 - 100 kDa. Tricine binds bivalent ions - particularly Cu2+- and interferes with the Lowry and BCA assay. Photooxidized by flavines. |
| A1379 | Tris 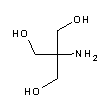 |
8.06 | 7.5 - 9.0 | -0.028 | Tris base. The most frequently used buffer in biological research. Most important applications: electrophoresis buffer TBE (e.g. A0972), TAE (e.g. A4227) and Laemmli buffer (A1415), DNA-stabilizing TE buffer (e.g. pH 8, A0973), TBS buffer for Western Blots and ELISA. The pH value strongly depends on temperature and decreases with dilution. Tris is a primary amine and may form Schiff bases with aldehydes/ketones. It inactivates DEPC and is involved in some enzymatic reactions (e.g. alkaline phosphatase). Please note that Tris is toxic for mammalian cells and should not be used in cell culture. |
| We provide Tris in various quality grades, as a 1M solution with defined pH as well as the hydrochloride (e.g. A1087). | |||||
| A1071 | 8.00 | 7.6 - 8.6 | -0.015 | Similar properties as HEPES. Due to a high buffer range suitable for photophosphorylation studies, especially if tricine (binds metal ions!) cannot be applied. Interferes with protein assay according to Lowry, but not with the BCA test. Can form radicals and is not suitable for redox studies. | |
| HEPPS | |||||
| A1024 | 8.26 | 7.6 - 9.0 | -0,018 | Zwitterionic, versatile "Good" buffer; used in buffers for enzyme reactions and electrophoresis. Suitable for low-temperature applications. Strong binding of Cu2+-ions; interferes with the BCA and Lowry protein assay. Ferricyanide slowly oxidizes bicine. | |
| A1141 | Taurine (AES) | 9.06 | 8.4 - 9.6 | -0.022 | Zwitterionic buffer. |
| A1065 | CHES | 9.50 | 8.6 - 10.0 | -0.011 | Suitable for crystallization of phosphotriesterase or chemical modification of bacteriorhodopsin. Interferes with protein assay according to Lowry. |
| A0838 | AMP | 9.69 | 8.7 - 10.4 | -0.032 | Well suited for the determination of enzyme activity, especially of alkaline phosphatase, lactate and malate dehydrogenase. |
| AMP has a low melting point and has to be liquified at approx. 35°C to prepare a buffer solution. The liquified AMP has a high viscosity, which makes its handling more difficult. If this buffer solution is stored at room temperature and protected from the CO2 from the air, it is stable for approx. one month. | |||||
| A1067 | Glycine 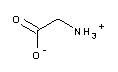 |
pK2=9.78 | 8.8 - 10.6 | -0.025 | Component of the Laemmli buffer (A1415) for SDS-PAGE of proteins; interferes with protein assays according to Lowry (>1mM) and Bradford (>100mM |
| A3900 | Carbonate | pK2=10.33 | 9.5 - 11.1 | -0.009 | Sodium carbonate |
| A1046 | Phosphate | pK3=12.33 | -0.026 | di-sodium hydrogenphosphate | |
| (Hydrogenphosphate) |
Legend
# changes in pK value per degree Celsius
§ 5 % CO2 atmosphere
Data partly taken from Bollag, D.M. & Edelstein, S.J. (1992) Protein Methods, Chapter 1, II (pp. 3-9). Wiley-Liss, New York.
Requirements of biological buffers
Originally, different inorganic substances were used as buffers, e.g. phosphate, cacodylate, borate and bicarbonate. Later also weak organic acids were used. Many of these buffer substances, however, have the disadvantage that they are not inert and have lasting effects on the system under investigation (e.g. inhibition of enzymes, interactions with enzyme substrates etc.). Most of the biological buffers in use today were developed by NE Good and his research team and are N-substituted taurine or glycine buffers. These zwitterionic substances largely meet the requirements or properties required for biological applications.
-

Solubility
The buffer should be freely soluble in water and poorly soluble in other solvents. The higher the water solubility, the simpler it is to prepare concentrated stock solutions.
-

Permeability
The buffer should not be able to permeate biological membranes to prevent an aggregation in the cell or organelles. Tris has a relatively high degree of liposolubility and may therefore permeate membranes. This also explains its toxicity for many mammalian cells in culture.
-

Ionic strength
The buffer should not significantly alter the ionic strength of the system. The physiological ionic strength is between 100 – 200 mM KCl or NaCl. This can be very important, especially when investigating enzymatic reactions, because the ionic strength of the solution is a measure of the ionic milieu, which may also affect the catalytic activity of an enzyme. The protonisationand deprotonisation depending on the ionic composition of the surrounding medium in the reaction set-up affects the binding and conversion of an enzyme substrate by the enzyme.
-

pKa value
The pKa value of a buffer should be influenced as little as possible by the buffer concentration, the temperature and the ion composition of the medium. Amongst the buffers with temperature dependent pKa values are the amine buffers, whilst carboxylic acid buffers generally react less sensitively to changes in temperature.
-

Complex formation
When a buffer forms complexes with metal ions, protons are released, which causes the pH value to decrease. The formation of insoluble precipitates usually represents a greater problem, however. If enzymes need the metal ions for their activity, these would be inhibited. Complexes should therefore be soluble and their binding constant should be known. Phosphates, for example, form insoluble salts with bivalent metals and precipitate. Phosphate buffered salt solution (PBS) is never autoclaved with Ca2+ or Mg2+ for this reason. Good buffers, such as PIPES, TES, HEPES and CAPS have very low metal-binding constants and are therefore particularly suited to investigate metal-dependent enzymes.
-

Inert substances
The buffer should not be subject to either enzymatic or non-enzymatic changes, i.e. it should not be an enzyme substrate or enzyme inhibitor and should not react with metabolites or other components. The buffer should therefore be inert. Phosphate and pyrophosphate are both substrates and inhibitors of different enzymatic reactions (inhibition of carboxypeptidase, urease, various kinases, various dehydrogenases). Borate forms covalent complexes with mono- and oligosaccharides, ribose subunits of nucleic acids, glycerol and pyridine nucleotides. Bicarbonate is in equilibrium with CO2 and therefore needs a closed system. Tris and other primary amines like glycine can form Schiff’s bases with aldehydes and ketones. They also interfere with the Bradford protein assay. Tricine is photooxidized by flavins, and daylight is therefore sufficient to reduce the activity of flavone enzymes. HEPES, HEPPS and Bicine interfere with Lowry protein assays. Buffers that are chemically based on the piperazine ring may form radicals under certain circumstances.
-

UV absorption
Buffers should not absorb any light at wave-lengths longer than 230 nm, since many spectrophotometric investigations are performed in this range (determination of the concentrations of DNA, RNA and proteins). ADA, for example, has an absorption of 0.1 at 260 nm. If buffers interfere with photometric analyses, they should be neutralised or set at the pH optimum for the test system used (Lowry pH 10; BCA pH 11; Bradford pH 1; colloidal gold pH 3). If this is not possible, proteins can be precipitated with trichloroacetic acid, perchloric acid or acetone, for example, and can then be redissolved in a solvent that does not interfere.
Criteria for the selection
Selection of the buffer for the correct pH range
The pKa value of the buffer should be in the range of the pH optimum for the test system. If the pH is likely to increase during the experiment, then a buffer should be chosen with a pKa value that is slightly higher than the optimum at the beginning of the experiment. Conversely, if the pH value is expected to decrease during the experiment, a buffer with a slightly lower pKa value should be chosen.
Determination of the pH optimum of an enzyme
If an enzyme is to be investigated, the first step is usually to determine the conditions under which the enzyme will show the highest possible degree of stability and activity. To determine the pH optimum it is advisable to initially use buffers that cover a broad pH spectrum, e.g. MES, PIPES, HEPES, TAPS, CHES and CAPS. Once the pH optimum has been identified, other buffers (e.g. for the pH value 7.5: TES, TEA or phosphate) can be tested, in order to be able to rule out or minimise non-specific buffer effects for later investigations. The pKa value of a buffer, i.e. the mid-point of its pH range, should be as near as possible to the desired pH value for the buffer being used. In other words, the pKa should correspond to the pH optimum of the enzyme under testing. The protonated (ionized) forms of amine buffers have less inhibitory effects than the non-protonated forms. For Tris and zwitterionic buffers, therefore, a working range slightly lower than the pKa value is usually more suitable, whilst in contrast to this, carboxylic acid buffers with a working range slightly above their pKa values are better suited, since these buffers consist mainly of the ionized form.
Determination of the optimum buffer concentration
An adequate buffer capacity is often only reached at concentrations higher than 25 mM. However, higher buffer concentrations and related high ionic strengths can inhibit enzyme activity. Suitable initial concentrations are therefore between 10 and 25 mM. If, after addition of the protein or enzyme, the pH value changes by more than 0.05 units, the concentration of the buffer can first be increased to 50 mM. Up to this concentration, no interference was observed with the Good buffers in cell culture experiments. In order to form complexes with heavy metals, EDTA can be added.
Application-dependent choice of buffer substances
The decision for or against a buffer is also dependent on the method for which it is used. In addition to the measurement of activity, the protein concentration is commonly determined after isolation or purification processes. Many of the buffer substances based on amino acids can lead to false-positive results in protein assays (e.g. Lowry) due to interactions with the reagents or absorption of the buffer substance itself in the range above 230 nm. Such interference can, however, easily be abolished by inclusion of the buffer in the blank control.
Many buffers are basically suitable for gel filtration. Cationic buffers such as Tris are preferred for anion exchange chromatography. Anionic buffers (such as phosphate or MES) should be preferred for cation exchange chromatography or hydroxylapatite chromatography, i.e. the buffer should have the same charge as the ion exchange material, to prevent it binding itself to the ion exchanger.
Borate is not suitable for the isolation of glycoproteins or systems that include nucleotides, since it interacts with the cis-hydroxyl group of sugars. If electrophoresis is performed subsequent to dissolution of the protein in protein purification systems, a buffer with a low ionic strength should be used, since a high ionic strength would heat up the gel.
The Good buffers based on the piperazine ring – HEPES, HEPPS, HEPPSO and PIPES – are not suitable for the investigation of redox processes, since, in the presence of H2O2, oxygen radicals, autooxidising iron or, under certain electrolytic conditions, they easily form radicals. In contrast to this, the Good buffer based on a morpholine ring, MES, does not form any radicals.
Setting the pH value
Buffers consist of an acid and its conjugated base. The quality of a buffer is determined by its buffer capacity, i.e. its resistance to changes in pH when strong acids or bases are added. In other words: the buffer capacity corresponds to the amount of H+ or OH– ions that can be neutralised by the buffer. The buffer capacity is related to the buffer concentration and achieves its maximum at the pKa value. This value corresponds to the mid-point of the pH range covered by the buffer (concentration of acid and base is equal). As a consequence, relatively large amounts of H+/OH– ions result in only small changes in pH. A buffer of a pH value one pH unit above or below its pKa value shows no buffer capacity any longer.
Calibration of the pH value is not that easy that it seems to be at first sight. There are a number of facts that influence the proton concentration and effect the buffer's functioning:
Acid/ Base: Commonly the pH value is set using NaOH/KOH or HCl. Slow addition of the acid or base while stirring vigorously avoids locally high concentrations of H+ or OH– ions. If this is not done, the buffer substances may undergo chemical changes that inactivate them or modify them so that they have an inhibitory action. If a buffer is available in the protonized form (acid) and the non-protonized form (base), it is recommanded to set the pH value by mixing the two substances.
If monovalent cations interfere with the reaction or are to be investigated, the pH value can be set with tetramethyl or tetraethylammonium hydroxide. Acetate, sulphate or glutamate can be used instead of HCl, although here the risk of interference with an enzyme is particularly high.
Temperature: Depending on the buffer substance, its pH may vary with temperature. It is therefore advisable to set the pH at the later used working temperature. The physiological pH value for most animal cells at 37°C is between 7.0 and 7.5. One buffer particularly susceptible to changes in temperature is Tris. If set to 7.5 at 37°C, it increases to about 8.5 at 0°C. Good buffers generally have a low degree of temperature sensitivity, and carboxylic acid buffers (citrate, formate, succinate) are even less sensitive.
Nowadays, many pH meters have an integrated function that enables setting of the pH value at room temperature and allows for different working temperatures (e.g. +4°C or +37°C). A limitation on this function, however, is that the dpKa/dT value, i.e. the value for the change in the pKa value (dpKa) dependent on the temperature change (dT), is not the same for all buffers. For example, the change in the pKa value for Tris with an increase of 1°C amounts to 0.028 units, whilst the value for HEPES changes by only 0.014. Imprecision is unavoidable with this approach, since such changes should actually be accounted for with the pH meter.
Buffer additives: If other components are added to the buffer (e.g. EDTA, DTT, Mg2+), changes in the pH should also be expected and it should be retested.
Ionic strength: The setting of the ionic strength of a buffer solution (if required) should be performed in the same way as the setting of the pH value when selecting the electrolyte. The ionic strength depends on the electrolyte. The salts of tetramethylammonium or tetraethylammonium are suitable for the setting of the ionic strength, since the larger cations do not interact so well with the negative charges of the enzymes. Acetate, as a large anion, has a poor interaction with the alkali metals.
pH meter control: Nowadays, accurate pH meters with a digital display are usually available for the setting of the pH value of a buffer. The pH meter is calibrated using two pH standards which cover the range of the buffer to be set. If there are any doubts about the precision of the device, this can simply be resolved by standardising the pH meter using 50 mM phosphate buffer, which is then diluted 10fold. The pH value should then be 0.2 pH units higher.