For longer than 100 years the Kjeldahl method has been used for the determination of nitrogen in a wide range of samples. The determination of Kjeldahl nitrogen is made in foods and drinks, meat, feeds, cereals and forages for the calculation of the protein content. Also the Kjeldahl method is used for the nitrogen determination in wastewaters, soils and other samples.
It is an official method and it is described in different normatives such as AOAC, USEPA, ISO, Pharmacopeias and different European Directives. The Kjeldahl method is used to determine the nitrogen content in organic and inorganic samples.
The procedure involves three major steps:
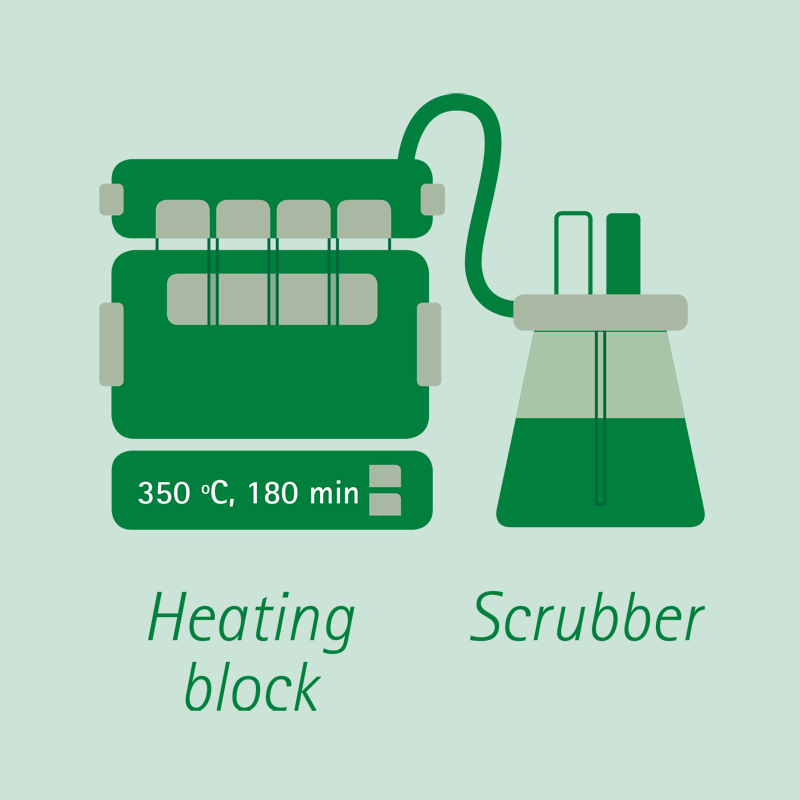
Digestion:
The sample is digested in boiling concentrated sulfuric acid, with the addition of a catalyst, until complete dissolution and oxidation. The nitrogen contained in the sample becomes Ammonium Sulfate.
| Protein (-N)+H2SO4 Sample |
-> | (NH4)2SO4+CO2+H2O Catalyst |
|---|
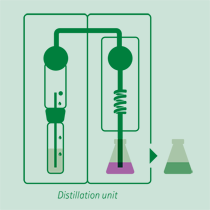
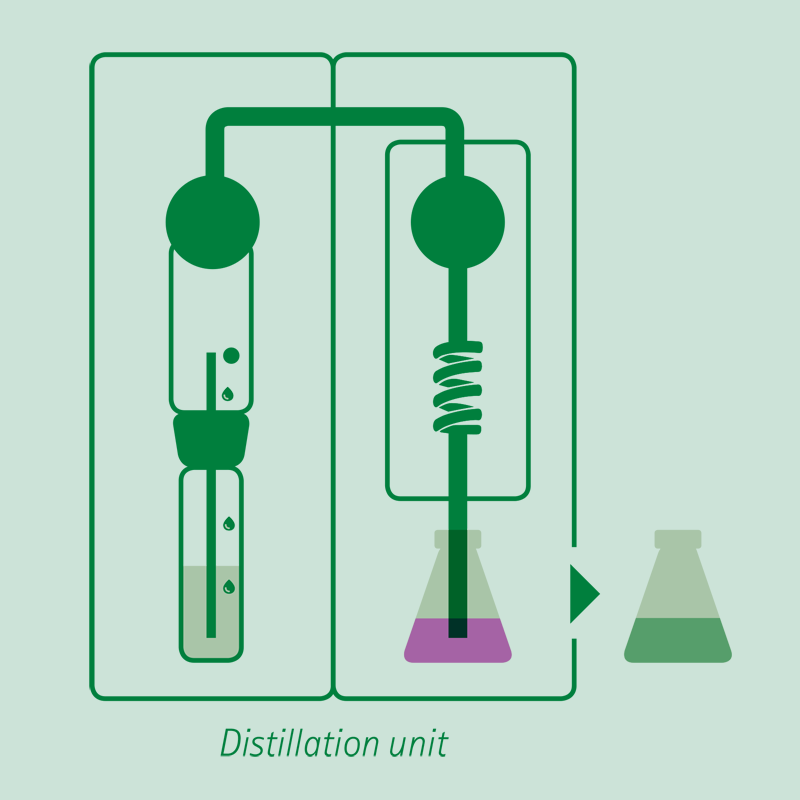
Distillation
Adding an excess of sodium hydroxide solution, the ammonium ion is released in ammonia form, distilled and received on a boric acid solution or a sulfuric or hydrochloric acid volumetric solution.
| (NH4)2SO4 + 2NaOH | -> | 2NH3 (gas) + Na2SO4 + 2H2O |
|---|
The receiving vessel for the distillate is filled with an absorbing solution in order to capture the dissolved ammonia gas.
- Common absorbing solutions involve aqueous boric acid [B(OH)3] of 2-4% concentration. The ammonia is quantitatively captured by the boric acid solution forming solvated ammonium ions.
B(OH)3 + NH3 + H2O = NH4+ + B(OH)4- - Also other acids can be used as precisely dosed volume of sulfuric acid or hydrochloric acid that captures the ammonia forming solvated ammonium ions.
H2SO4 (total) + 2NH3 -> SO42- + 2NH4+
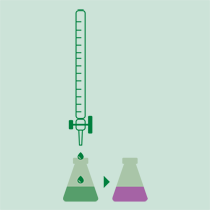
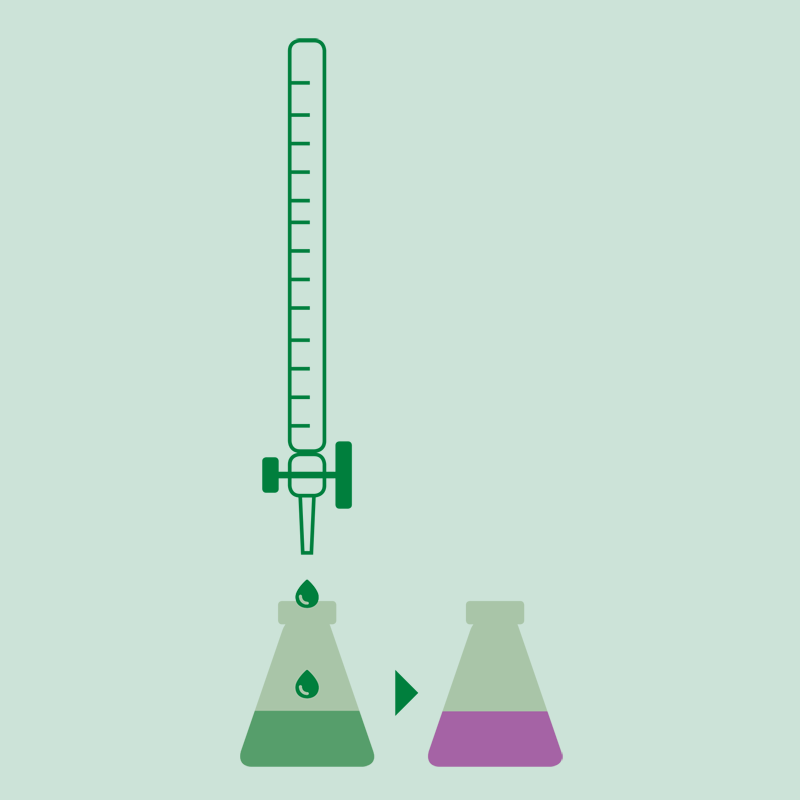
Titration
The ammonia is determined with a volumetric acid solution or by back titration with sodium hydroxide solution of a known concentration if it was received on hydrochloric or sulfuric acid. The results can be expressed in % N, % NH3 or protein (%N x factor).
- When using the boric acid solution as absorbing solution, an acid-base titration is performed using standard solutions of sulfuric acid or hydrochloric acid and a mixture of indicators.
B(OH)4- + HX = X- + B(OH)3 + H2O
HX= strong acid (X=Cl-, etc.) - When using sulfuric acid standard solution as absorbing solution, the residual sulfuric acid (the excess not reacted with NH3) is titrated with sodium hydroxide standard solution and by difference the amount of ammonia is calculated.
H2SO4(total) + 2NH3 -> SO42- + 2NH4+